Господдержка предприятий-производителей строительных материалов
The question of chemical resistance can only be considered from the point of view of finished products: during their processing, storage or use. In the overwhelming majority of cases, the most relevant are undesirable reactions of glass with water. Additional influence can be exerted by such atmospheric components as carbon dioxide, sulfur oxide, nitrogen oxide, special products of evaporation or combustion accompanying production.
The appearance of defects in the interaction of clean dry gases with glass is almost unbelievable; it takes moisture for this to happen.
The occurrence of defects resulting from the reaction of glass with solids is most likely in cases where a pulverized mixture or alkaline raw materials (for example, soda) is deposited on the glass and exposed to it in the presence of moisture or directly, during subsequent annealing at high temperatures ...
Reasons for the chemical activity of the glass surface
The regularities of changes in the physicochemical properties of silicate glasses can be logically explained on the basis of existing concepts on the structure of glass. One of the main factors affecting the properties of glass is the degree of connectivity of the silicon-oxygen framework and the activity of the oxygen ion in the glass. Silicon oxide (SiO2) exists in glass in the form of various types of [SiO4] tetrahedra. These tetrahedra should have different properties, depending on the amount of oxides of alkali (Me2O) and alkaline earth (MeO) metals in the glass, since bridging (doubly connected) oxygen differs in properties from nonbridging (simply connected) oxygen. In other words, the properties of [SiO2] tetrahedra should depend on the degree of connectivity of the silicon-oxygen framework. A measure of the degree of connectivity is the coefficient fsi, which is equal to the ratio of the number of silicon atoms to the number of oxygen atoms (Si / O), or the reciprocal, the oxygen number R = O / Si.
The fsi coefficient is calculated by the formula:
FSi = VSiO2: (VМе2О + VМеО + 3VМе2О3 + 2VМеО2 + 5VМе2О3 + 3VМеО3)
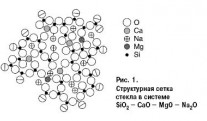
For a pure silica glass structure composed entirely of [SiO4] tetrahedra, each of the oxygen vertices is common to two neighboring tetrahedra. Each SiO2 molecule consists of one silicon ion and four halves of doubly connected oxygen ions equally spaced from Si4 +. When the oxides Ме2О or МеО are introduced into quartz glass, one vertex at a part of the (SiO4) tetrahedra is “weakened”, because in the vicinity of oxygen ions are ions Ме2 +, 1 +, which have a weaker field than Si4 +. The number of such tetrahedra increases with an increase in the content of Ме2О and МеО in the glass. This process of changing the structure of the silicon-oxygen framework is reflected in the properties of SiO2 in glass and, consequently, in the glass itself. The state of structural oxygen in the glass and its activity depend on the degree of connectivity of the silicon-oxygen framework. According to the theories of Zachariasen and Warren, the network of SiO2 tetrahedra is broken by the incorporation of sodium or calcium ions, and the rupture occurs along non-bridging oxygen ions. During the glass molding process, a large amount of alkaline ions is found on the surface of the molten glass. This “unoccupied alkali”, the amount of which can increase due to slow annealing (Me ions come from the inner glass layers), as well as “non-bridging” oxygen ions are the main reason for the chemical reactivity of the glass surface. In fig. 1, the relative sizes of the circles of these ions correspond approximately to the ratio of the ionic radii.
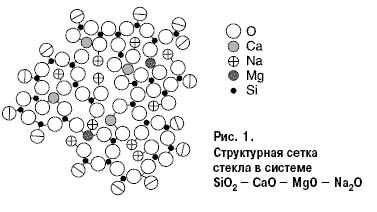
The eightfold coordination of calcium, and the fourfold coordination of magnesium, including two sodium ions necessary for the equalization of valence, are indicated as swarming. By projection on the plane, each tetrahedron becomes a triangle, each octahedron - a quadrangle. Oxygen ions at the border of the mesh are halved. If we imagine that the right boundary of the scheme is a surface, then we can see that with five silica molecules with non-bridging oxygen ions, two alkaline ions are energetically ready for chemical interaction.
The principle of the chemical interaction of glass with the participation of water
All chemical changes to glass surfaces are to be understood as reactions of end-elements of glass with unsaturated valence, such as silicon or coordination unoccupied sodium and potassium.
For fresh glass surfaces, for example, after its destruction, the bridge bond between silicon ions is broken, and two differently charged unstable “radicals” are formed:

These "radicals" are highly reactive towards the molecules of the medium in which they are found, especially water:
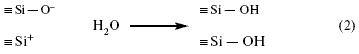
This means that in both cases the silicon atom combines with the OH group.
Reaction (2) can be represented as follows:
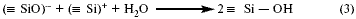
The Si - OH group is called silanol. The formation of bridging oxygen can be traced from reaction (2):

Silanol groups are chemically reactive: the hydrogen ion H moves relatively easily (alkaline ions associated with oxygen, such as sodium, lithium, are more mobile) and can be replaced by other compounds. Other oxides and, under certain conditions, whole layers can appear on the glass surface in this way. Then water molecules are adsorbed, a state of equilibrium with water vapor of the surrounding atmosphere is formed. The chemically bonded layer of water has a thickness
10-10 cm and is called "permanent hydrated surface layer", as opposed to "temporary hydrated surface layer", which is adsorbed physically in equilibrium depending on the moisture content in the air and has a thickness of approximately 10-7 cm.
The chemical interaction of the glass surface with other components always occurs with the participation of water or its decomposition products:

The quantitative relationship of this equation is given by the equilibrium constants according to the law of mass action:

This means that the concentration of hydrogen ions H + in an aqueous solution should increase if the concentration of OH– ions decreases, and vice versa.
The mechanisms of water (hydrolytic) and acid corrosion are very similar: in both cases, modifier cations are affected, especially reactive alkaline ions.

Both equations show that sodium ion is less strongly bound to oxygen than hydrogen ion. This fact underlies the processes of ion exchange in aqueous solutions known as leaching.
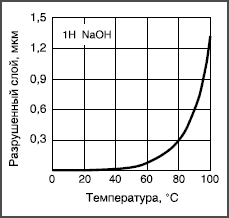
Figure: 2. Temperature dependence of the solubility of borosilicate glass in 1 N NaOH solution.
If hydrolytic and acid corrosion in ordinary glasses is insignificant, then alkaline solutions strongly corrode almost all glasses, dissolving even their grid. Corrosion intensity increases exponentially with alkali concentration and temperature (Fig. 2). The reason for this intensity of alkaline corrosion is that OH hydroxyl groups can split the oxygen bridge bond between two silicon ions:
It is not the emerging groups that react here, but the internal network structure is destroyed, i.e. the glass dissolves. For example, silica in alkaline solutions transforms into soluble silicate, aluminum - into aluminate, etc.
Chemical defects on the glass surface
It is necessary to distinguish between the behavior of aqueous media with excess and lack of moisture. If there is a lack of water (condensation), the reaction is possible at the moment of the appearance of moisture. As a result, the transformation of an acidic medium, which was formed during the adsorption of atmospheric gases (SO2, CO2), into an alkaline medium is quite likely due to the dissolution of glass components in water according to equations (4) and (7).
Excess water
Only in this case it is water that acts on the glass, since its amount exceeds the amount of reaction products, which, moreover, are removed from the reaction site immediately after formation. As already noted, this reaction “leaches out” the surface layers of the glasses; the reactive and soluble glass components pass into the solution. First of all, these are alkali ions. Divalent ions of alkaline earth elements react much more slowly, because aluminum and silicic acid are much stronger bound to the silicate framework.

Figure: 3. Hydrolytic processing of borosilicate glass depending on the time.
The thickness of the leached layer is calculated from the amount of released Na2O.
Leaching of the surface is proportional to the time t1 / 2. The graphical representation of the dependence of the amount of leached material on time at the beginning has the form of a parabolic curve (Fig. 3a). If t1 / 2 is plotted on the abscissa axis, then the dependence is rectilinear (Fig. 3b). With prolonged hydrolytic exposure, equilibrium is established between the glass surface and the aqueous medium, and the dissolution process stops (Fig. 4 and 5). This is a general pattern of the leaching process for all types of glass that do not completely dissolve.

Figure: 4. Achieving an equilibrium state during leaching of potassium-strontium
silicate glass.
The data are given: on the ratio of K and Si ions that have passed into the solution per day.

Figure: 5. Change in composition in corrosive layers enriched in silicic acid
a - a thin but stable layer;
b - thick unstable layer with a lower SiO2 content
Upon completion of the reaction with the alkalis on the surface, the interaction is transferred to the underlying layers, overcoming the obstacle of the silicate lattice, and therefore, the further process is diffusion. The diffusion rate at low (up to about 80 ° C) temperatures is relatively low. According to equation (2), silanol groups are formed on the surface. This process is the more intense, the more alkalis the glass contains. The layer formed during the reactions gradually thickens. Porosity and surface area increase accordingly. Such a surface is capable of adsorbing water and physically as a result of the action of capillary forces, and the leached surface layers swell. They got their own name: silicic acid gel layer. From a chemical point of view, they are identical to the protective layers.
If soda-lime or borosilicate glasses are used in accordance with their purpose, then a shallow gel layer forms on their surface, indicating the insignificance of the leaching effect. In this case, the transparency of the glass is completely preserved, and the reacted layer is not visible without the use of special optical means.
A gel layer up to 100-200 nm thick can be seen with the naked eye: it reflects light differently than the original glass. By changing the refractive index, the mirror effect is reduced. Gray prints appear on leached glass. However, as long as the surface layer does not interfere with normal viewing, it is not yet a glass defect.
With a further increase in thickness, the leached layer becomes white and transparent; if it is porous enough, then leaching can develop deeper, the layers will flake off, and the glass will gradually collapse (Fig. 6).
The leached glass surface is capable of attaching Cr (VI), Al (III) or Fe (III) ions, which are clearly visible due to their color and cannot be separated even by aqueous solutions of acids. If the silica gel layer is dried, only physically adsorbed water will be released, while the chemically adsorbed remains bound as a permanent water shell (Fig. 7).

Figure: 6. Glass with strongly swollen layers: at the top - the heating is weaker; below - stronger

Figure: 7. Glass leached with water and became "rough" after annealing (surface crystallization)
If the glass surface interacted not with chemically pure water (that is, distilled), but with ordinary tap water, then during drying, traces of impurities of elements that determine the hardness of water - Ca and Mg carbonates - are found in the sediment. If such a surface is subjected to drying at 120 ° C, then the impurities can intensively react with the glass up to the formation of spots. They cannot be removed by conventional cleaning methods or even with acids.
Lack of water
In the presence of a very small amount of water, the course of the reactions changes significantly. However, glass can always react with moisture present in the atmosphere. Water can also be released from raw packaging material (wood contains up to 60% water). This action is especially serious in humid climates. Condensation may form on glass sheets that are very cold at night. As shown earlier, the initial hydrolytic interaction with pure water becomes alkaline. Since hydroxyl ions act more strongly than water molecules, then according to equations (9) and (10), the released OH– ions increase the chemical activity. Since strongly alkaline solutions are hygroscopic, i.e. pull away moisture, then new portions of moisture can be absorbed from the atmosphere. As a result of water adhesion, thin alkaline films can form, which have a negative effect on surface properties. In the transition to alkaline action, the silica frame is torn, and the glass is destroyed. In the case of reactions with a lack of water, it is thus a question of the successive interaction of glass with the products of its own decomposition.
Gross hydrolytic defects occur when glassware packed in paper or cardboard is stored in a humid atmosphere. The packaging material absorbs moisture and creates conditions for the initiation of hydrolytic reactions. Often, certain components of the impregnation dissolve, accelerating the process. Typical hydrolytic interactions with wet packaging material are so-called paper prints (Figure 8). Methods for eliminating such defects are still unknown.
An interesting phenomenon was noted when talc was sprayed between sheets of glass. With moisture, it forms a kind of binder, as a result of the release of alkalis and silicic acid from the glass. This material, when clumping (for example, due to shaking during transportation), adheres relatively tightly to the glass (Fig. 9) and forms the so-called worm-like formations, which, however, can be easily removed with alkaline detergents.

Figure: 8. Paper prints

Figure: 9. Worm-like formations on sheet glass after glass sprinkling with talc
A vivid example of a hydrolytic reaction occurring with a lack of water is the action of moisture condensed between sheets of glass adjacent to each other in a stack, as a result of which a defect may form later on. Calculation shows that when 1 m3 of air saturated with moisture is cooled from 35 to 25 ° C, 7.5 g of water can be released. A drop of water weighing 50 mg will spread over the surface of the glass sheet over an area of approximately 50 cm2 (with a layer thickness of 0.05 mm). Under these conditions, the previously described reactions may well occur, which give corrosion spots in the first stage. Under certain conditions, they can be transparent. With prolonged interaction of glass surfaces, accompanied by condensation, corrosion, evaporation, etc., a compound such as sodium soluble glass is formed:
As a result of this process, the interaction of glass surfaces can be so intense that when trying to separate them, the glass will break.
Loss of glass due to adhesion occurs when glass sheets packed for long-term storage or transportation are in close contact. The foregoing explains the seemingly paradoxical position that sheet glass, absolutely reliable in glazing, is in danger under conditions of long-term storage in a packed form.
In case of hydrolytic action with a lack of moisture (for long periods of time) and interaction with carbon dioxide in the air, complete extraction of CaO from glass in the form of soluble hydrocarbonates is likely. The hydrolytic action can be reduced by the use of active media or by the formation of protective layers of silica gel. This can also happen if, as a result of interaction with the air atmosphere, new compounds such as calcite or gypsum (CaSO4 :) are formed.
If, after hydrolytic action with a lack of moisture, the reaction products are removed from the surface layers by excess moisture (rain), then the usual hydrolytic reaction will cause the formation of a silicic acid gel layer. A swollen layer is created, which, due to fluctuations in temperature and humidity, dehydrates again and over time, changing chemically and physically, reaches the thickness visible to the eye when its size exceeds 1/4 of the wavelength of visible light: then thin films with an iridescent border are visible.
Coloring occurs due to the phenomenon of interference of visible light on the destroyed surface layers. The first signs of deterioration are a slight decrease in light reflectance compared to the non-corroded surface, which is slightly colored (gray to brown). With further thickening of the layer, for example, “blue spots” appear (Fig. 11). As the hydrolysis intensifies, the layer scatters light more and finally becomes white. For the most part, it has the appearance of a cloudy or spotty haze, and in extreme cases, the layer can flake off with scales.

Fig. 10. The formation of an etched stain between two sheets of glass as a result of the formation of hydroxyl ions on the surface and, as a result, the formation of an alkaline solution in place
contact of sheets.

Figure: eleven.
Blue spots on the mirror glass
The surface hydrolyzed layers consist of silica gel, which changes continuously when the reaction products are removed (the appearance of the layer is similar to that obtained during hydrolytic treatment with excess water); if the reaction products are not removed, then they are deposited either in the thickness of the layer or on its surface. The pore depth in such a SiO2 body is 50-120 A °. The presence of an open pore structure can be proved by the fact that when dyes are introduced into the layer, its color changes.
In fig. 12 shows an exemplary scheme of glass reactions with a lack of water, when the reaction products remain on (or in) the surface layer.

Figure: 12. Scheme of interaction of glass and air moisture
(lack of water)
A special form of destruction of sheet glass is the so-called filamentous crystals, or “crow's paw”, which are formed by the reaction of acidic oxides of air (CO2 and SO2) with alkalis released during the decomposition of glass. Filamentous crystals are composed of carbonates. These crystals, grown oriented in accordance with the direction of glass stretching, are shown in Fig. 13. They appear on condition that the glass is first moistened and then dried. They can often be observed even before the first turbidity. They indicate the beginning of decomposition, although they are not yet a defect of the glass surface, but only warn of the possibility of its appearance.

Fig.13.
Thread crystals or “crow's paw” formed under various conditions of interaction of glass with air oxides (CO2 and SO2).
It is possible to avoid defects such as "turbidity" and others on an industrial scale by using solutions of zinc nitrate at elevated temperatures (60-100 ° C). Such processing of glass should more than 3 times increase the resistance of glass compared to untreated glass.
Exposure to acids
Excess aqueous acid solutions
Equation (8) characterizes the process of acid action: replacement of alkali metal ions with the formation of silanol groups. To eliminate, as far as possible, the release of the ions under consideration, for example, from laboratory glassware, which can seriously interfere with analytical work, the method of leaching the glassware with hot acids is used (“aging” of laboratory glassware).
Hot concentrated phosphoric and hydrofluoric acids are capable of destroying glass, acting on it at any concentration, due to the formation of readily soluble compounds with the silicon ion. Borofluorosilicic acid reacts in the same way.
Chemical etching, mainly by means of solutions containing hydrofluoric acid or alkali, also increases the mechanical strength of the glass by eliminating surface defects.
Exposure to gaseous media
Acid gas traces always appear in the area where solid fuels, combustible gases or fuel oil are burned. Heavy fuel oils contain a significant amount of sulfur, which forms oxides when burned. Each glass product is exposed to high temperatures from the moment of its formation, as well as during annealing. Alkaline ions react primarily with traces of acids and acid anhydrides of the furnace atmosphere, especially with sulfur oxides and carbon dioxide. The reaction in the presence of traces of moisture for SO2 can be represented as follows:

In this way, salts are formed, which create a sulfurous coating on the surface of the products. Since in this process alkalis in the surface layer of the glass are neutralized, this plaque plays the role of protecting the surface during annealing. A decrease in the proportion of fuel oil as a fuel for glass-melting furnaces and the transition to electric furnaces for annealing glass exclude the formation of sulfurous deposits.
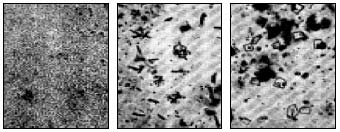
Fig. 14. Micrographs of a sulfurous deposit in various stages, crystallized under the influence of air moisture
Gaseous products contribute to the production of most of the sodium compounds on the glass surface, which dissolve easily in water. Therefore, this plaque on products should not be considered a defect at all - it can be easily removed by rinsing with water. Sulfurous deposits can change their appearance as a result of recrystallization or hydration, in particular, due to moist air; sometimes, to start recrystallization, the moisture that is communicated to the glass when you touch it with your hand is enough. In fig. 14 shows photomicrographs of a sulfurous deposit, which crystallized under the action of moisture in the exhaled air.
Exposure to alkalis
Under natural conditions, alkaline reactions are possible only when hydroxyl ions reacting with a lack of moisture are selected in accordance with equation (11) and act as alkalis. Exposure to alkalis in excess is possible only under specially created conditions.
Excess alkali
Under the action of alkalis, the Si-O-Si bonds of the silicon-oxygen framework of the glass are destroyed due to dissolution reactions proceeding according to equations (9) and (10). With an increase in the temperature and concentration of OH- ions, the process intensifies at first exponentially and later linearly (Fig. 15). This mechanism takes place at pH = 10.
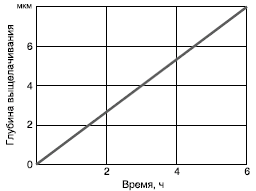
Figure: 15. Dependence of the depth of the leached layer on the duration of treatment of borosilicate glass with alkalis (1 N NaOH solution, T = 100 ° C)
Such reactions occur when the initially neutral medium becomes alkaline due to the absorption of OH- ions. This case has already been discussed in detail earlier.
Specificity of chemical interactions of the surface of float glass
The composition of float glass and the batch used for its melting belongs to the group of classic cast and drawn glasses, for which sodium sulfate is usually used for clarification.
Contact of hot glass melt with tin melt and with a reducing atmosphere under normal conditions does not affect the quality of the finished glass, although the composition of the glass surface differs markedly from its bulk. Between the Na2SO4 contained in the glass, as well as the hydrogen (10%) contained in the protective atmosphere of the float bath, and the molten tin, chemical reactions take place with the release of volatile compounds: SnS, Na2S and H2O. As a result of these reactions, the glass surface facing the protective atmosphere of the bath is depleted in Na +, Ca2 +, Mg2 +, (SO3) 2– ions.
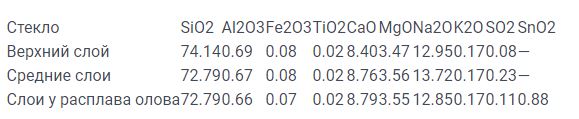
The removal of Na + from the surface in contact with the tin melt is facilitated by the fact that a certain amount of tin participates in the antidiffusion in the form of Sn2 +. In addition, part of the water chemically dissolved in the glass is released from both sides of the tape into the very dry atmosphere of the float chamber. Compositional changes are limited by the thickness of the surface layer of the glass from 0.01 to 0.02 mm and depend on the duration of the stay of the glass in the float bath, and therefore, they increase with increasing glass thickness. Differences in the composition of six-millimeter glass by tape thickness are shown in Table 1.
Table 1. Difference in chemical composition by thickness of float glass tape
I.A. Kumalagov
PhD in Engineering Science